Many bacteria secrete secondary metabolites to compete or cooperate with other microbes or hosts in diverse and dynamic ecological niches. 2-Phenylethanol (2-PE) and indole-3-acetic acid (IAA) are small metabolites that play important roles in biological and ecological functions, produced by microorganisms. They are synthesized via expanded shikimate pathways, and required the key enzyme α-ketoacid decarboxylase. Here we show an adaptive strategy driven by secondary metabolites in accordance with bacteria survival state. A soil derived Enterobacter strain CGMCC 5087 produces 2-PE in exponential growth phase whenever in nutrient rich or limited environments that suppresses microbial competitors, but produces IAA at the onset of stationary phase only in a tryptophann rich environment enabling plant growth promotion, which is in an α-ketoacid decarboxylase KDC4427 dependent manner. The metabolic fluxes of 2-PE and IAA are mediated by the ratio of KDC4427 and an L-glyceraldehyde 3-phosphate reductase gene ADH4428, which are transcribed divergently by a bidirectional promoter in one operon, and by the enzyme activity characteristics of KDC4427. The expression of KDC4427 is up-regulated with bacteria growth, while ADH4428 is down-regulated; simultaneously, KDC4427 shows a higher kcat value for phenylpyruvate, and has a higher affinity for indolepyruvate, thus making the reaction flow towards the production of 2-PE in exponential growth phase, however as the growth of bacteria enters the stationary phase, the production of IAA is increased. Additionally, we demonstrated that TyrR and RpoS activate and repress the expression of KDC4427 and ADH4428 through direct binding to the bidirectional promoter. These results reveal an ingenious control of competition and cooperation behaviours through fine-tuning the sequential synthesis of 2-PE and IAA in response to growth and environmental conditions.
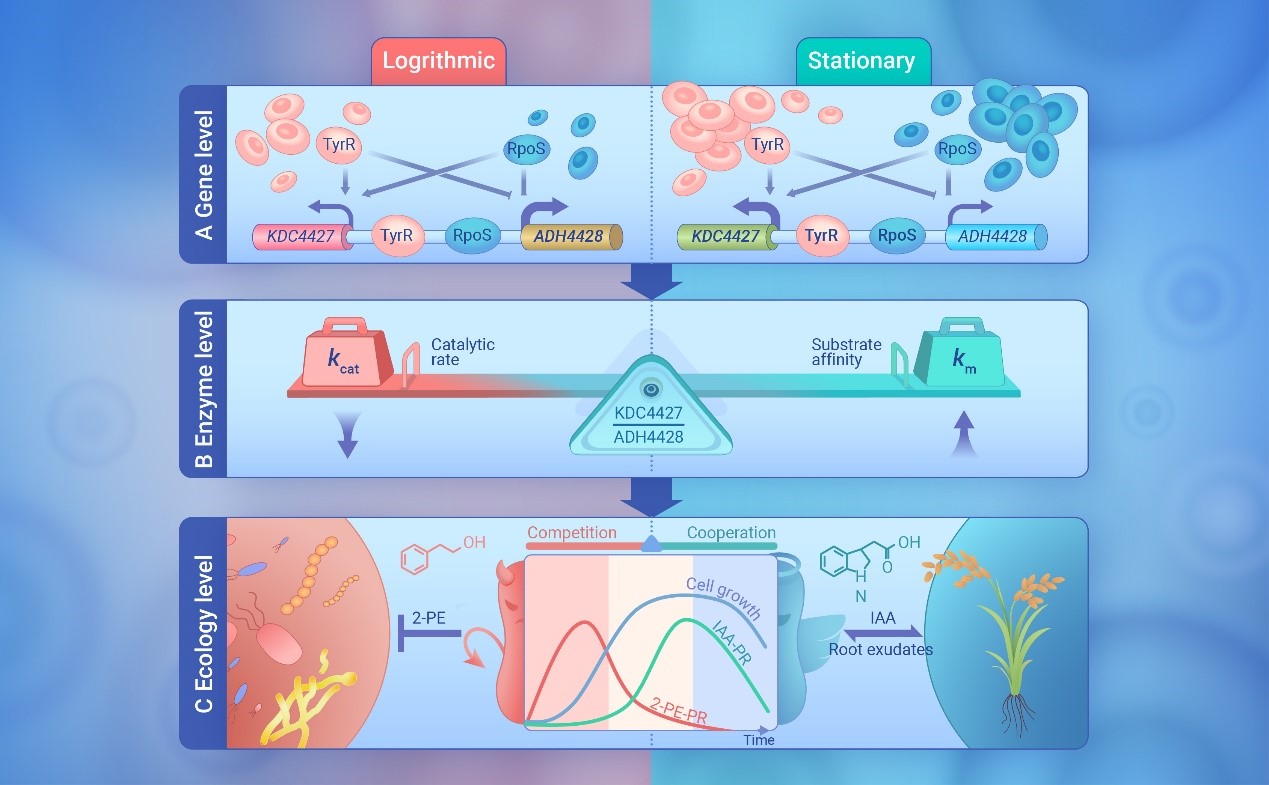
This work reveals an intriguing possibility that Enterobacter sp. CGMCC 5087 has potential application in agriculture for plant health and growth. It may contribute to a better understanding of the interaction of bacteria and their habitat as well as aid in metabolic engineering. The work is supported by National Natural Science Foundation of China, Shandong Provincial Science Fund for Distinguished Young Scholars, Young Taishan Scholars, Dalian National Laboratory for Clean Energy (DNL), CAS; QIBEBT I201934, and China Postdoctoral Science Foundation.
Link: https://doi.org/10.59717/j.xinn-life.2023.100023
